Lynx.MD Proudly Supports Prometheus Lab’sCrohn’s Colitis Congress 2024 Poster Presentation
Lynx.MD is honored to be a part of Prometheus Lab’s research presented at the Crohn’s & Colitis Congress in January 2024. The study, led by presenter Andrew Shim, delves into the realm of therapeutic monitoring and its impact on real-world outcomes in the treatment of Inflammatory Bowel Disease (IBD).
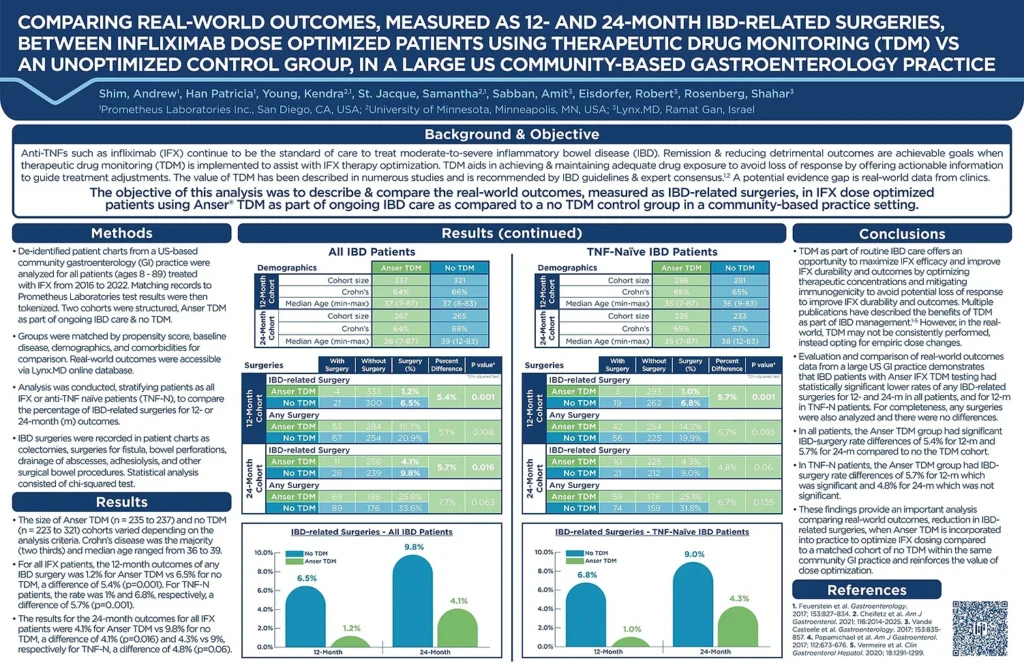
Click to enlarge. Comparing Real-World Outcomes, Measured as 12 And 24-Month IBD-Related Surgeries, Between Infliximab Dose Optimized Patients Using Therapeutic Drug Monitoring (TDM) Vs An Unoptimized Control Group, In A Large US Community-Based Gastroenterology Practices. Presented at Crohn’s & Colitis Congress, January 2024.
Platform
Prometheus Labs
Lynx.MD Trusted Data Environment Research Platform
Gastro Health Longitudinal Patient Data Source
Strategic Differentiation
Prometheus Laboratories worked in partnership with the Lynx.MD data science team to define and analyze the data.
With the Lyxn.MD Trusted Data Environment the longitudinal patient data remained in Gastro Health’s secure cloud environment. All data was anonymised prior to study.
The Lynx.MD platform provided statistical insights on the fly and tailored specific filtering functionality – which enhanced the speed to value approach for Prometheus research team in fine tuning protocol details.
Contributors
- Andrew Shim, Presenter, Medical Affairs, Prometheus Laboratories Inc, San Diego, CA, University of Minnesota, Minneapolis, MN
- Patita Han, Research & Development, Prometheus Laboratories Inc, San Diego, CA
- Kendra Young, University of Minnesota, Minneapolis, MN
- Samantha St. Jacque, University of Minnesota, Minneapolis, MN, Prometheus Laboratories, San Diego, CA
- Amit Sabban, Data Analytics, Lynx.MD, Ramat Gan, Israel
- Dr. Robert Eisdorfer, Chief Medical Officer, Lynx.MD, Ramat Gan, Israel
- Shahar Rosenberg, Data Science and Analytics, Lynx.MD, Ramat Gan, Israel
- Gastro Health, Data Partners, Miami, FL
Abstract
The study examines the impact of therapeutic drug monitoring (TDM) with Anser® on optimizing Infliximab (IFX) therapy for moderate-to-severe IBD in a community-based gastroenterology setting. It aims to compare real-world outcomes, including IBD-related surgeries, between IFX dose-optimized patients using Anser® TDM and an unoptimized control group.
Methods
De-identified patient charts from Gastro Health, a US-based community gastroenterology practice, treated with IFX from 2016 to 2022, were analyzed. The Anser TDM cohort and no TDM control group were matched by propensity score, baseline disease, demographics, and comorbidities. Real-world outcomes were accessible via the Lynx.MD secure cloud-based data environment, with statistical analysis conducted by Lynx.MD.
Results
The Anser TDM group demonstrated significantly lower rates of IBD surgeries at both 12 and 24 months compared to the no TDM group. For all IFX patients at 12 months, rates were 1.2% vs. 6.5% (P=0.001), and at 24 months, 4.1% vs. 9.8% (P=0.016). Similar trends were observed in TNF-N patients.
Conclusions
This analysis provides crucial insights demonstrating that the Anser IFX TDM group exhibited significantly lower rates of IBD surgeries compared to a matched cohort of no TDM patients in the same community gastroenterology clinic setting.

